Gold is a chemical element with the symbol Au and atomic number 79. It is a dense, soft, malleable and ductile metal with a bright yellow color and luster, the properties of which remain without tarnishing when exposed to air or water. 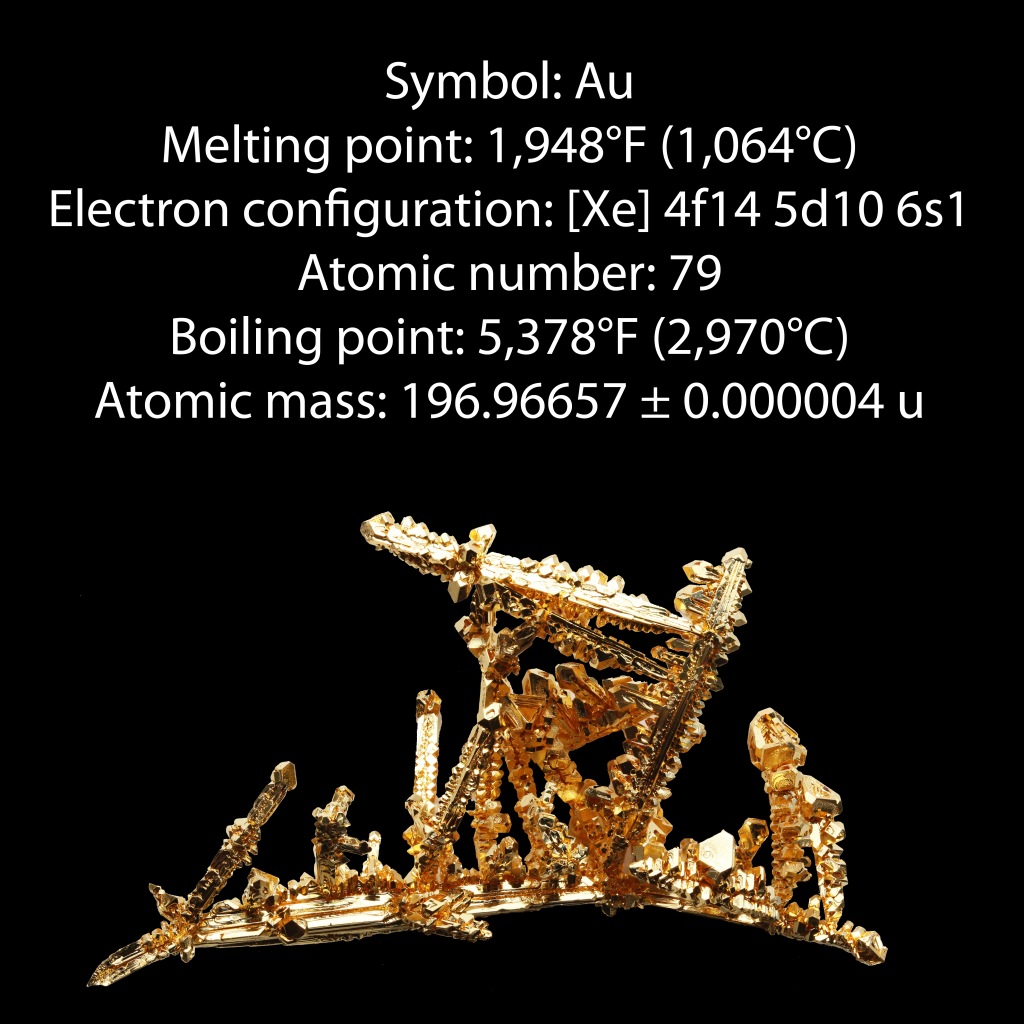 Chemically, gold is a transition metal and a group 11 element. It is one of the least reactive chemical elements, and is solid under standard conditions. The metal therefore occurs often in free elemental (native) form, as nuggets or grains, in rocks, in veins and in alluvial deposits. Less commonly, it occurs in minerals as gold compounds, such as with tellurium as calaverite, sylvanite, or krennerite.
Chemically, gold is a transition metal and a group 11 element. It is one of the least reactive chemical elements, and is solid under standard conditions. The metal therefore occurs often in free elemental (native) form, as nuggets or grains, in rocks, in veins and in alluvial deposits. Less commonly, it occurs in minerals as gold compounds, such as with tellurium as calaverite, sylvanite, or krennerite.
As the metallic native element mineral, gold structurally belongs to the isometric copper group. It also forms a solid solution series with the native element silver (Ag) to which it is often naturally alloyed (electrum). Other common natural gold alloys are with copper and palladium (Pd).
Gold resists attacks by individual acids, but it can be dissolved by aqua regia (nitro-hydrochloric acid), so named because it dissolves gold. Gold also dissolves in alkaline solutions of cyanide, which have been used in mining. It dissolves in mercury, forming amalgam alloys; it is insoluble in nitric acid, which dissolves silver and base metals, a property that has long been used to confirm the presence of gold in items, giving rise to the term acid test.
This metal has been a valuable and highly sought-after precious metal for coinage, jewelry, and other arts since long before the beginning of recorded history. The value of gold is rooted in its medium rarity, easily handling, easy smelting, non-corrosive, distinct color and non-reactive to other elements; qualities most other metals lack.
Besides its widespread monetary and symbolic functions, gold has many practical uses in dentistry, electronics, and other fields. Its high malleability, ductility, resistance to corrosion and most other chemical reactions, and conductivity of electricity have led to many uses, including electric wiring, colored-glass production, and gold leafing.
The asteroid that formed Vredefort crater 2.020 billion years ago is often credited with seeding the Witwatersrand basin in South Africa with the richest gold deposits on earth. However, the gold bearing Witwatersrand rocks were laid down between 700 and 950 million years before the Vredefort impact. These gold bearing rocks had furthermore been covered by a thick layer of Ventersdorp lavas, and the Transvaal Supergroup of rocks before the meteor struck. What the Vredefort impact achieved, however, was to distort the Witwatersrand basin in such a way that the gold bearing rocks were brought to the present erosion surface in Johannesburg, on the Witwatersrand, just inside the rim of the original 300 km diameter crater caused by the meteor strike. This brought their rich gold deposits to the notice of humans in 1886, and launched the Witwatersrand Gold Rush. Nearly 50% of all the gold ever mined on earth has been extracted from these Witwatersrand rocks.
I don’t know about you but this is the kinda thing that I love investing in and owning. How about you?